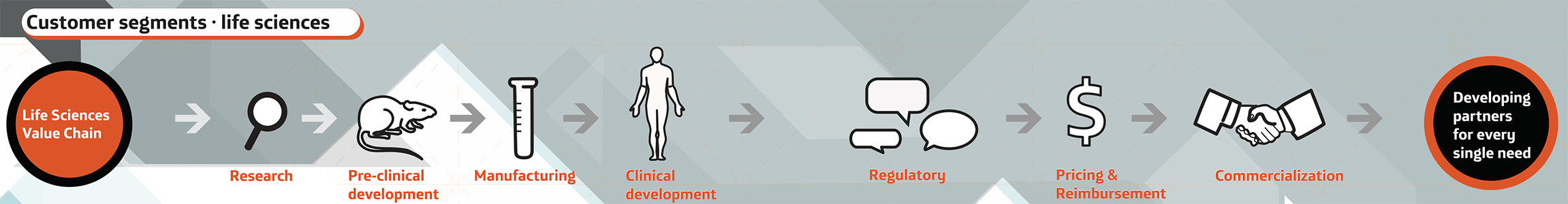
Since 2006, Tecnología Innovativa provides services to companies seeking business partnerships in Argentina and Latin America, promoting projects across the life sciences value chain.
Our company
Tecnología Innovativa was originated in the Academia. Founded by a team of professionals with extensive experience in the development of new pharmaceutical technologies and new bussiness development in the pharmaceutical industry.
Our staff includes scientists, market and business specialists in pharma industry, clinical research and development of new molecules.
We have former experience working at leading companies in the pharmaceutical business, holding positions in different areas including R&D, market research, product development, new business development, sales and marketing.
Since 2006, Tecnología Innovativa provides services to companies seeking business partners in Argentina and Latin America, promoting various projects across the chain of value in life sciences.
Hover
Tecnología Innovativa has strong and trusting bonds with leading companies in the Argentine and Latin American pharmaceutical market and a proven track record in generating successful agreements of licensing with subsequent successful product launches.
Tecnología Innovativa has a strong force in Argentina and Latin America by partnering with local companies. Our partners and distributors are mature organizations which provide integral support for companies looking to enter LATAM market. This includes:
Description of services we provide:
Tecnología Innovativa, are currently working together with academics centers to promote research, diagnosis, and development of new treatments, focusing on treatments for people with rare genetic diseases of low incidence, or special treatments of low incidence and high cost.
Together, they offer the possibility to pharmaceutical companies, currently working in the research and development of drugs for rare and special therapeutic areas, of support and knowledge to be introduced at the Latin American market, through local research, promotion of diagnosis, and selection of local partners who can enhance and facilitate the launch of new Specialty Products.
Tecnología Innovativa S.A. is dedicated to providing solutions to the pharmaceutical industry. We offer a range of services tailored to their needs, focusing on clinical studies of bioequivalence, bioavailability and pharmacokinetics, and in vitro biowaivers of In Vivo Bioavailability and Bioequivalence Studies (using cultured epithelial cell monolayer) ( *FDA, 2000. Guidance for industry: Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate Release Solid Oral Dosage Forms based on Biopharmaceutics Classification System. US Food and Drug Administration, Center for Drug Evaluation and Research, USA).
We have a team of academic experts in Medical Pharmacology of the School of Medicine, University of Buenos Aires, national reference center in pharmacology. We have a strong interdisciplinary group of professionals with extensive experience in clinical research, basic research and analytical techniques, including medical doctors, molecular biology and biochemistry, all specializing in pharmacology.
Clinical trials and bioequivalence studies are conducted according to the regulations established by ANMAT and in compliance with good clinical practice guidelines (defined in ICH E6: Guideline for Good Clinical Practice), Declaration of Helsinki and the Nuremberg Code. Clinical research is conducted at a private hospital of high complexity, in which we have an area reserved for healthy volunteers, an area of hospitalization and adult intensive therapy of high complexity, surgery rooms equipped with the latest technology and a very prestigious team of medical professionals.
The bioanalytical quantification phase is developed in specialized centers ANMAT certified and with extensive experience in the field.
DOSSIER ASSEMBLY SERVICES: Our team has extensive experience in the bibliographic assembly of modules 4 and 5 of the Dossier for registration in CTD format.
Please fill the form to contact us.